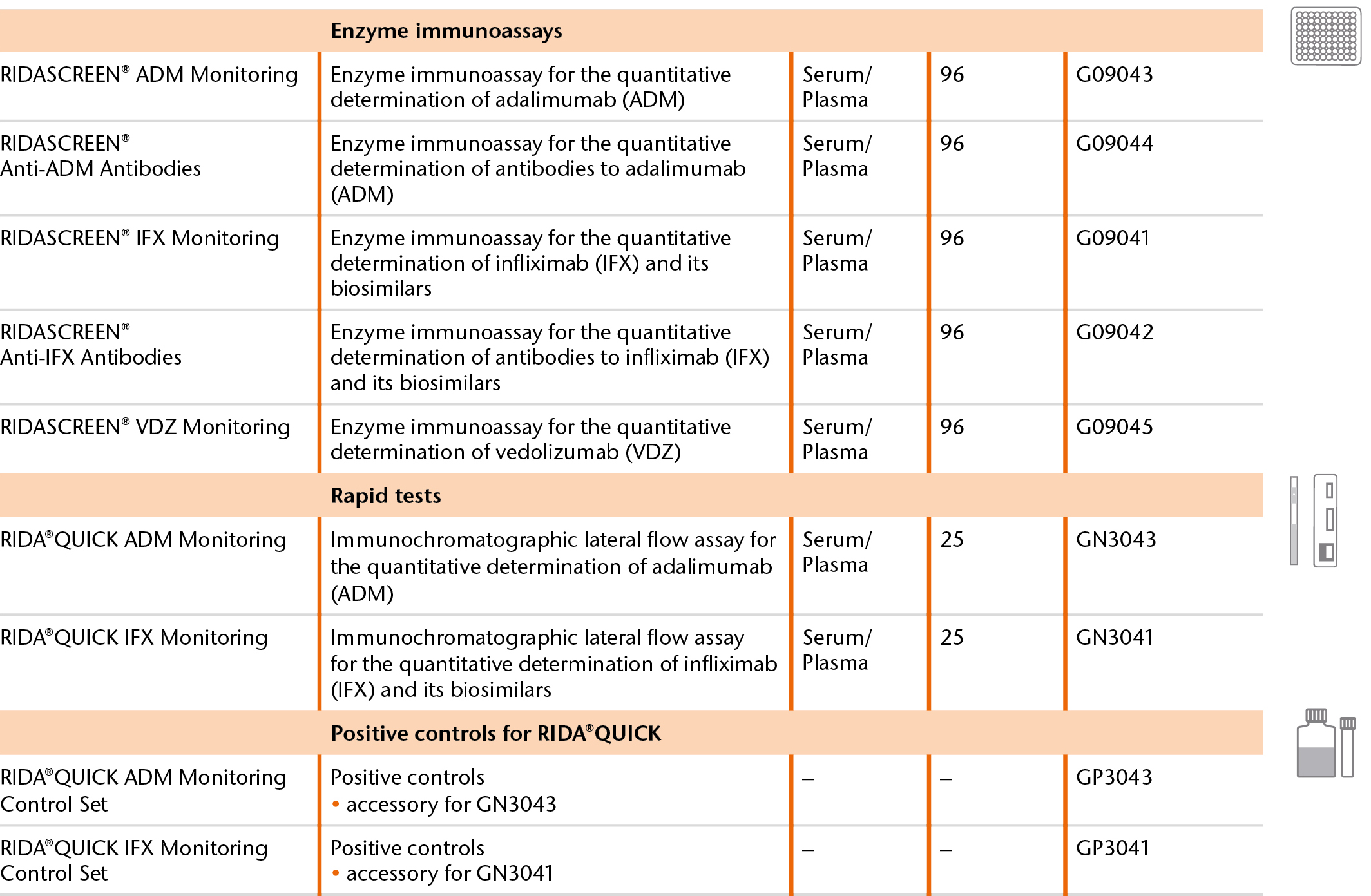
Chronic inflammatory diseases like Crohn’s disease, ulcerative colitis, and rheumatism respond well to treatment with the TNF-α blockers infliximab and adalimumab. Monitoring the concentration of the active ingredient in the patient’s blood is recommended to avoid over- and underdosing and ensure successful treatment over the long term. R-Biopharm offers an extensive range of products to help.
The new RIDA®QUICK ADM Monitoring adalimumab rapid detection test expands the product portfolio for therapeutic drug monitoring (TDM). The range of rapid detection and ELISA tests keeps medical practices and laboratories alike optimally prepared. All test systems contain ready-to-use reagents and are validated by the University of Leuven in Belgium.
Immunochromatographic lateral flow tests are suitable for quick monitoring directly in the office. The quick tests from R-Biopharm identify the trough level in only 20 minutes for immediate dose adjustment. They are available for measuring infliximab and its biosimilars as well as for adalimumab. Testing is extremely easy and requires minimal lab equipment, as the following video shows.
ELISA tests support efficient analyses even with high specimen volumes. The analyses can also be performed automatically using ELISA equipment (e.g., DSX®). The portfolio for therapeutic drug monitoring includes tests to determine infliximab, adalimumab and vedolizumab. In some cases, patients develop antibodies to the drug that neutralize its effect. At very low trough level concentrations, the blood should therefore be tested for anti-drug antibodies to determine if therapy requires adjusting. These tests are also available.
Our portfolio at a glance: