With our ELISA tests and our lateral flow test for therapeutic drug monitoring (TDM), you can check the drug concentration in the patient’s blood in order to adjust the dosage to the individual needs of the patient.
So called TNFα blockers are biologic agents which are used for the treatment of inflammatory diseases such as ulcerative colitis, Crohn’s disease and rheumatoid arthritis. Infliximab (IFX, Remicade®, Remsima™, Inflectra®) as well as Adalimumab (ADM, Humira®) belong to this group of drugs.
Video: Therapeutic Drug Monitoring explained
With the R-Biopharm test kits for therapeutic drug monitoring (TDM), you can check the drug concentration in the patient’s blood. This video explains why this is important.
Interactive: TDM Checker
The TDM Checker is an interactive tool helping you to make the right therapy decisions based on the measured drug concentrations.
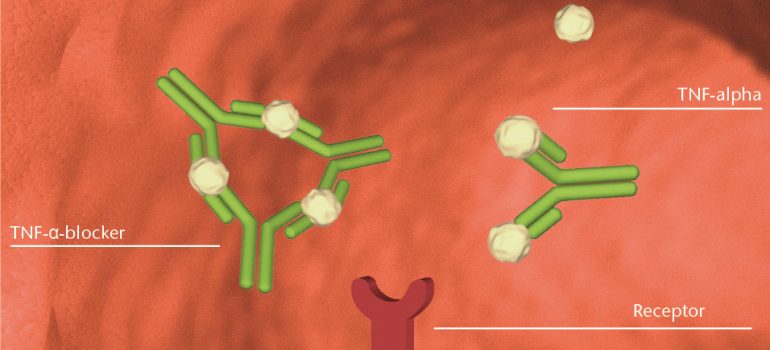
How do TNFα blockers function?
When an inflammation starts, cells of the immune system increase the production of TNFα. TNFα binds to specific receptors, which trigger the signal for the inflammatory process.
In case of chronic inflammation, the immune system continuously produces TNFα, so that the inflammation does not cease. TNFα blockers interrupt this circle by binding to the messenger TNFα. Hereby the molecule can no longer bind to the receptor, so that the signaling cascade of the inflammatory process is interrupted. As a result, the inflammation and its symptoms can decrease or cease.
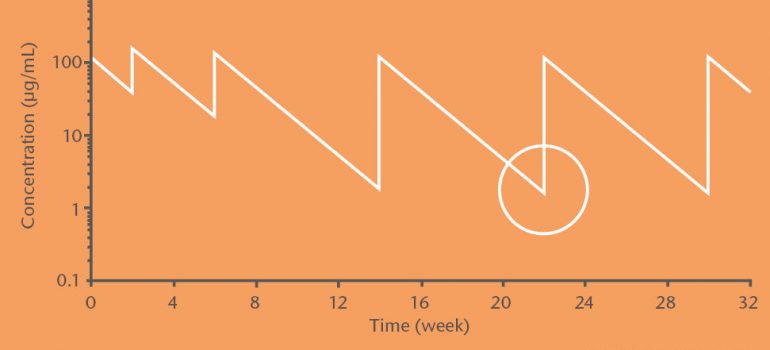
Individual dose adjustment by measuring drug levels and immunogenicity
In order for TNFα blockers to work optimally, it is important to check their trough level on a regular basis, since the bioavailability differs from person to person. The trough level (TL) is defined as the drug concentration in the blood measured right before the next infusions.
Moreover, immunogenicity has an impact of the efficacy of the drug. So called anti-drug antibodies (ADA) bind to the drug and can lead to a decrease in drug availability in the metabolism as well as to allergic reactions. Monitoring of drug and anti-drug antibody-levels of TNFα blockers helps to optimally adjust the therapy to the individual needs of the patient.
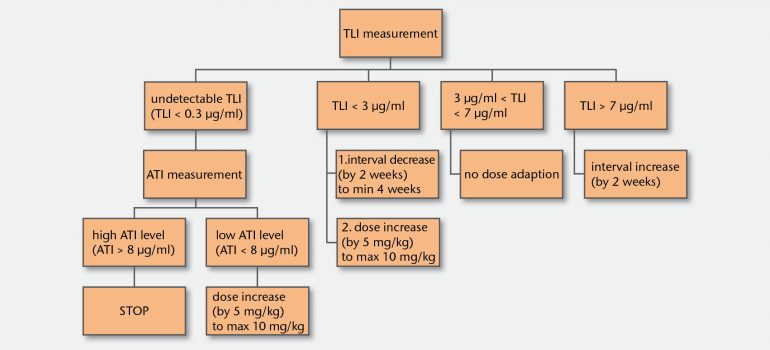
Therapy adjustment based on therapeutic drug monitoring
The TAXIT algorithm (TAXIT = trough concentration adapted infliximab treatment) is a recommendation for therapy adaptation based on the results of through- and anti-drug-antibody levels of Infliximab. It is a result of the study by Nils Vande Casteele et al. (KU Leuven) which investigated the effect of drug monitoring on the outcome of TNFα treatment.
The study shows the positive effect of TDM for therapy optimization and treatment cost reduction. Moreover, it indicates that testing for anti-drug antibodies does not have to be performed on a standard basis but only in patients with undetectable trough level of Infliximab. RIDASCREEN® IFX Monitoring and RIDASCREEN® Anti-IFX Antibodies are based on the assays used in this study.
Downloads
You can find more information on our test kit portfolio for therapeutic drug monitoring in our free customer brochures:
Choose a technology
Choose your TDM product
You might also be interested in
TDM support
Questions? Tap into our team’s expertise. We’re here to support you and your business throughout the testing process to ensure your success