Intended use:
For in-vitro diagnostic use. RIDA®GENE STI Mycoplasma Panel is a multiplex real-time PCR for the direct, qualitative detection of Mycoplasma hominis, Mycoplasma genitalium and Ureaplasma urealyticum/parvum from human genital swabs and urine.
The RIDA®GENE STI Mycoplasma Panel multiplex real-time PCR is intended for use as an aid in diagnosis of urinary tract infections or infections of the genital area caused by Mycoplasma hominis, Mycoplasma genitalium and Ureaplasma urealyticum/parvum.
General information:
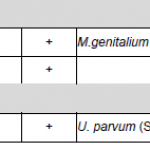
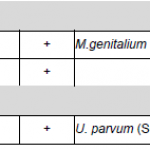
Mycoplasma species may persist as part of the normal human flora of the respiratory system or the genital area. Of seven known mycoplasma species from the genital area, so far only four have been described as pathogenic: Mycoplasma hominis, Mycoplasma genitalium, Ureaplasma urealyticum und Ureaplasma parvum.
Mycoplasma hominis (M. hominis) mainly colonizes the genital tract of sexually active men and women, however most of the M. hominis described infections have been diagnosed in woman. M. hominis is associated with pelvic inflammatory disease (PID) and may cause infections during or after pregnancy, such as endometritis or neonatal pneumonia. Common symptoms for infections with M. hominis include e.g. frequent urination, yellow discharge or dysuria.
Globally, the prevalence of Mycoplasma genitalium (M. genitalium) ranges between 1- 4 % for men and 1 – 6.4 % for women. In men, M. genitalium may result in non-specific urethritis and is the second most common cause after Chlamydia trachomatis. About 30 % of persistent urethritis is linked to M. genitalium. In women, M. genitalium infections may lead to cervicitis, endometritis, urethritis or pelvic inflammatory disease (PID).
Ureaplasma urealyticum (U. urealyticum) and Ureaplasma parvum (U. parvum) are parasitic, gram-negative bacteria which can be part of the urogenitalflora of men and women. In 2002, the earlier existing nomenclature of 14 U. urealyticum serovars has been updated, so that serovar 1, 3, 6 and 14, which groups to Parvo Biovar (Biovar 1), are now listed as separate species (U. parvum). Serovars 2, 4, 5, 7, 8, 9, 10, 11, 12 and 13 belong to T960 Biovar (Biovar 2) and therefore are listed as U. urealyticum. In women, U. urealyticum prevalently causes pelvic inflammatory disease (PID) and Ureaplasma colonizes the vaginal flora of up to 50 % of pregnant women. During pregnancy Ureaplasma may be transmitted to the child, which can lead to pneumonia or diseases of the central nervous system.
Image Gallery
| Art. No. | PG4945 |
|---|---|
| Test format | real-time PCR with 100 reactions |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.