Tuberculosis remains the world’s leading infectious disease killer.
WHO – Global tuberculosis report 2024

In 2023, 10.8 million people fell ill with Tuberculosis and 1.25 million lost their lives.
(WHO – Global tuberculosis report 2024)

Latent Tuberculosis affects approximately one-quarter of the global population, with individuals at risk of progressing to active Tuberculosis.

Early and accurate detection and treatment of Tuberculosis is crucial to breaking the chain of transmission and eliminating Tuberculosis globally.
Tuberculosis diagnostic solutions based on the new marker IP-10
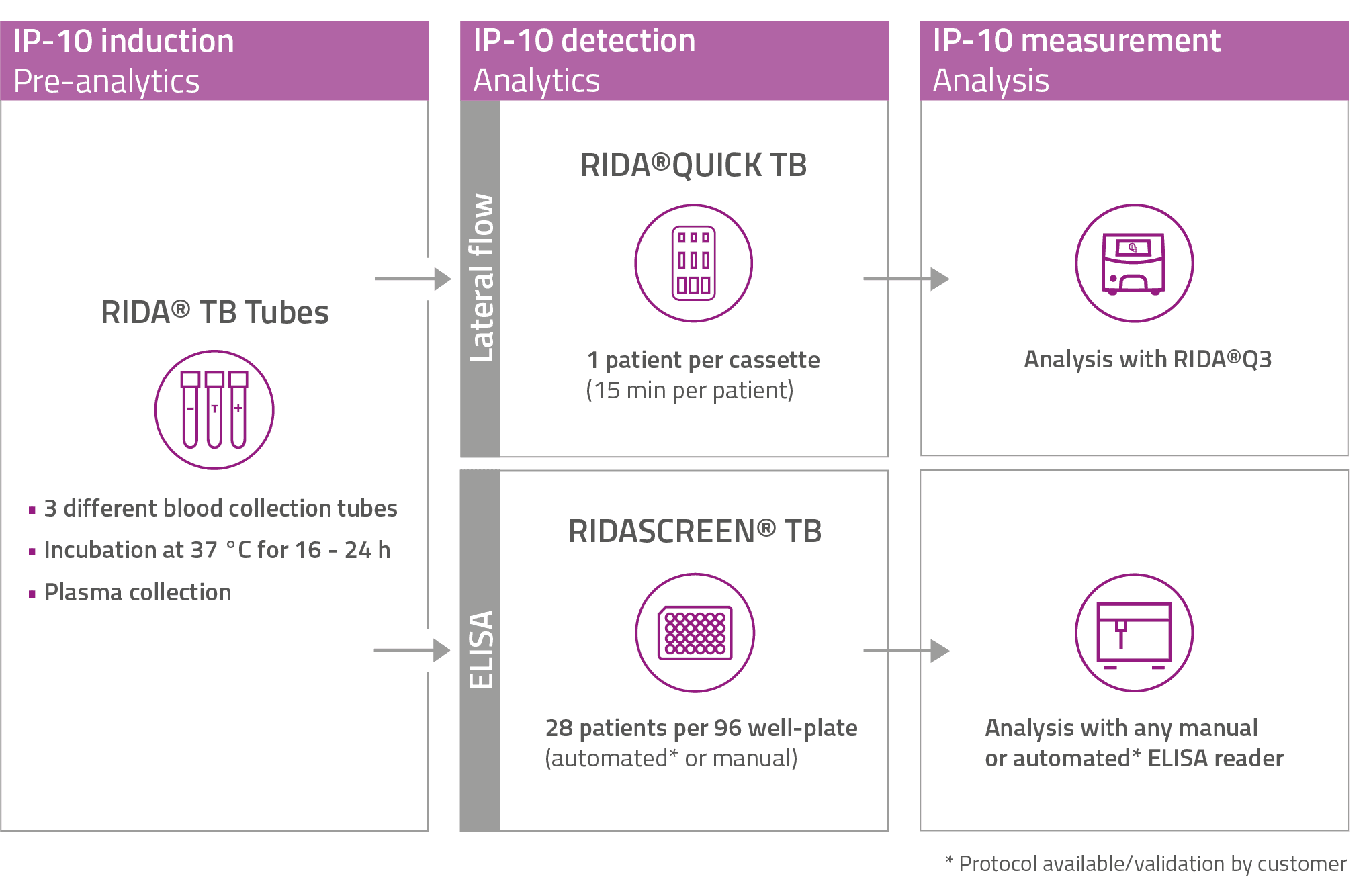
It just takes three stimulation tubes and one of the associated tests – either ELISA or lateral flow – to tackle Tuberculosis (TB) in all environments from fully equipped laboratory set-up to remote locations.
R-Biopharm’s TB diagnostics (CE IVDR approved) are based on the detection of the biomarker IP-10 (interferon-gamma induced protein 10) which is secreted in concentrations of up to 100 times higher than interferon-γ after specific T-cell stimulation with tuberculosis antigens. IP-10 is a chemokine that is released in response to interferon-γ and plays an important role in the inflammatory response.
This new generation of TB diagnostics therewith marks a major leap forward from traditional latent TB testing. It provides healthcare professionals with simple, reliable, efficient and easily accessible diagnostics for every setting.
IP-10 and TB
- Established marker: IP-10 is a well-studied biomarker with numerous publications demonstrating its association with TB.
- Comprehensive expression profile: IP-10 is directly related to interferon-γ release. It is not restricted to T-cells activation, but also produced by other immune cells, resulting in significantly higher concentrations.
- Sensitivity: The advantageous expression profile of IP-10 can contribute to improved sensitivity, particularly in challenging cohorts.
Products

 RIDA® TB Tubes (CE IVDR approved)
RIDA® TB Tubes (CE IVDR approved)
- Only 3 sterile induction tubes per patient
- 3 vacuum blood collection tubes for direct blood collection (negative control, TB-specific test tube, unspecific positive control)
- ESAT-6 and CFP-10 as established and specific TB-antigens
- IP-10 induction in just 16 h
- Up to 100-fold induction compared to interferon-γ.
- Minimized waiting times

 RIDA®QUICK TB (CE IVDR approved)
RIDA®QUICK TB (CE IVDR approved)
- Lateral flow test – optimal for decentralized testing
- One test cartridge per patient for straightforward handling
- Results in just 15 min, allowing immediate decision-making
- Robust colloidal gold technology, less sensitive to light exposure, ensuring reliability in all settings (no need for fluorescence)
- Easy read-out and result interpretation with RIDA®Q3 reader (continuous loading)

 RIDASCREEN® TB (CE IVDR approved)
RIDASCREEN® TB (CE IVDR approved)
- ELISA test compatible with manual & automated* workflows
- Up to 28 determinations per plate
- For medium to high-throughput settings
- Minimized waiting times
- All required reagents included in one kit for seamless implementation
* Protocol available/validation by customer
IP-10 assay workflow
Performance
Performance compared to QuantiFERON®-TB Gold Plus (QFT-Plus).
Multicentric diagnostic study with 339 patients from 7 study centers in Germany and South Africa.
85.2 % sensitivity in active TB patients vs 71.7 % QFT-Plus
- PPA and NPA with QTF-Plus
- Positive percent agreement 96.9 %
- Negative percent agreement 86,9 % *
- Strong agreement between RIDA®QUICK TB and RIDASCREEN® TB
- Positive percent agreement 92,1 %
- Negative percent agreement 98,9 %
* Negative percent agreement lower due to higher sensitivity of the RIDASCREEN® TB in clinically confirmed active TB patients
Contact us for more information
You are currently viewing a placeholder content from HubSpot. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information





