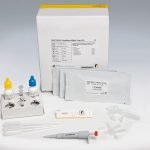
Intended use:
For in vitro diagnostic use. RIDA®QUICK Clostridium difficile Toxin A/B is an immunochromatographic rapid assay for the qualitative determination of the toxins A and B of Clostridium difficile in stool samples and in culture supernatants.
General information:
The increase in frequency and increasing severity of Clostridioides (Clostridium) difficle infection (CDI) leads to rising healthcare expenses, and increased morbidity and mortality. The implementation of multistage testing algorithms has improved overall diagnostic accuracy being of crucial importance for therapy decisions.
RIDA®QUICK Clostridium difficile Toxin A/B supports CDI detection:
- Direct determination of free C. difficile toxin A and toxin B in the patient specimen
- Elaborated antibody mixture for high specificity
- Obtains reliable results in combination with RIDA®QUICK Clostridium difficile GDH
- Clear result readout with colloidal-gold particles
Image Gallery
Accessories:
| Art. No. | N0803 |
|---|---|
| Test format | 25 tests |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.