Intended use:
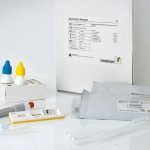
The RIDA®QUICK Norovirus test is a quick qualitative immunochromatographic test for determining
genogroup 1 (GG I) and genogroup 2 (GG II) noroviruses in stool samples. It is used as an aid in the diagnosis of gastroenteritis and for the analysis of stool samples from children and adults with the symptoms of suspected gastroenteritis caused by the norovirus.
General information:
Noroviruses are single stranded RNA viruses that cause norovirus gastroenteritis worldwide.
Noroviruses are frequently involved in outbreaks in communal facilities, such as nursing homes or hospitals, and sporadic gastroenteritis cases. Moreover, this pathogen is responsible for most gastroenteritis cases not caused by bacteria.
Norovirus infections are known to have a considerable impact on public health being able to cause severe staff shortages and unnecessary antibiotic treatments. Rapid diagnostic testing assist health care professionals to identify rapidly outbreaks and improve patient management.
Excellent and fast norovirus detection with RIDA®QUICK Norovirus:
- Elaborated composition of norovirus-specific antibodies enables reliable detection
- Proven quality for determining the antigens of both norovirus genotypes I (G1) and II (G2) (1,2)
- Individual determinations for low and irregular sample throughput enable fast infection control
Image Gallery
Accessories:
| Art. No. | N1402 |
|---|---|
| Test format | Rapid test cassettes consisting of 25 tests |
| Incubation time | 15 min test run |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.