Intended use:
For In-vitro diagnostic use. Enzyme linked immunosorbent assay (ELISA) for the quantitative determination of human pancreatic elastase in feces as an aid in the diagnosis of the exocrine pancreatic function.
General information:
Pancreatic Elastase is a proteolytic enzyme exclusively produced in pancreas. It is stable during the intestinal passage and is even accumulated in a sixfold concentration in stool, compared to the concentration in the duodenal juice. The widely utilized ELISA for the quantitative determination of pancreatic elastase in feces enables to obtain a quick and reliable diagnosis and follow-up of an exocrine pancreatic insufficiency, which may be caused by:
- Chronic pancreatitis
- Cystic fibrosis
- Diabetes mellitus
- Cholelithiasis
- Hereditary Pancreatitis
- Chronical- inflammatory bowel disease
- Pancreatic carcinoma
- Autoimmunologically caused pancreatitis
- Shwachman-Diamond-Syndrome
- Zollinger-Ellison-Syndrome
Image Gallery
Accessories:
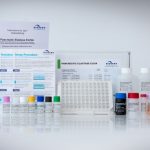
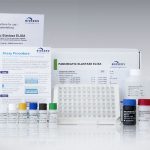
| Art. No. | G09040 |
|---|---|
| Test format | Microtiter plate with 96 wells (12 strips with 8 breakable strips each) |
| Standard range | 5 Standards (15 - 50 - 100 - 200 - 500 µg Elastase/g stool) |
| Incubation time | approx. 140 min |
| Cut-off | – severe exocrine pancreatic insufficiency 200 µg elastase/g stool |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.