Intended use:
For in vitro diagnostic use. RIDA®GENE PVL is a real-time PCR for the direct, qualitative detection of the PVL-gene (Panton-Valentine leukocidin) from cultures.
RIDA®GENE PVL real-time PCR is intended for use as an aid in diagnosis of (skin- / soft tissue-) infections caused by PVL.
General information:
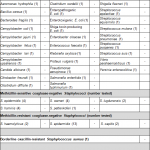
Staphylococcus aureus is a normal commensal of the skin and mucous membranes of humans that can be detected in 30% of the general population (asymptomatic carriers). Strains of Staphylococcus aureus which have developed antibiotic resistance methicillin and narrow-spectrum ß-lactamase resistant penicillin antibiotics are called MRSA (Methicillin-resistant Staphylococcus aureus). Staphylococcus aureus is a major cause of nosocomial infections in hospitals and healthcare settings.Since the mid-1990s the number of infections in the population increased with no previously history of medical facilitiy contact. This increase in infections in the population is caused by Staphylococcus aureus strains that carry the virulence factor Panton-Valentine leukocidin.
PVL can be produced by methicillin-sensitive Staphylocoocus aureus strains (MSSA) as well as and methicillin-resistant Staphylocoocus aureus strains (MRSA). MRSA strains that carry the virulence factor PVL are called CA-MRSA. Panton-Valentine leukocidin (PVL) is a bicomponent, poreforming cytotoxin encoded by the lukF-PV and lukS-PV genes. The cytotoxin of PVL lysis macrophages as well as neutrophil granulocytes and contributes to tissue necrosis. The clinical manifestion of PVL-positive Staphylococcus aureus strains are skin and soft tissue infections, particularly recurrent invasive abscesses. Rarely necrotizing pneumonia develops with a mortality rate of up to 75%. Risk groups for transmission CA-MRSA or PVL-MSSA are for example families, persons performing close contact sports, persons from educational settings, prisoners and military personnel. In the recent years infections with PVL-MSSA and PVL-MRSA increased worldwide.
Advantages
- Fast lysis possible
- PCR results in less than 1.5 hours
- Ready-to use reagents
- Harmonized Workflow and PCR profile allows parallel processing of multiple RIDA®GENE assays (DNA and RNA assays)
- All components included, also Lysis Buffer, Internal Control, Positive Control and Negative Control
Image Gallery
Accessories:
| Art. No. | PG0645 |
|---|---|
| Test format | 100 reactions |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.