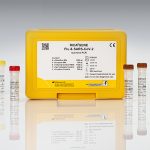
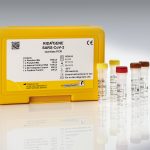
Intended use:
For in vitro diagnostic use. The RIDA®GENE Flu & SARS-CoV-2 test, performed on the LightCycler® 480II real-time PCR instrument, is a multiplex real-time RT-PCR for the direct qualitative detection and differentiation of flu A/flu B and coronavirus (SARS-CoV-2) RNA in human nasal/throat swabs from persons with signs and symptoms of respiratory infection.
The RIDA®GENE Flu & SARS-CoV-2 test is intended to support the differential diagnosis of flu A/flu B and SARS-CoV-2 infections in patients with symptoms of respiratory infection in connection with other clinical and laboratory findings.
Negative results do not rule out infection with flu A/flu B or SARS-CoV-2 and should not be used as the sole basis for diagnosis. The product is intended for use by professionals working in hospital laboratories, reference laboratories, private laboratories, or public laboratories.
Detects also the new Omicron variant (B1.1.529)!
General information:
At the end of December 2019 in the Chinese metropolis of Wuhan, numerous cases of pneumonia of unknown cause occurred. At the beginning of January 2020, Chinese authorities identified a novel coronavirus (SARS-CoV-2) as the cause of these illnesses. The disease caused by SARS-CoV-2 was officially named
COVID-19 (“coronavirus disease 2019”) and is transmitted from human to human. Due to this pathogen’s novelty, the epidemic rapidly evolved into a pandemic.
There have already been 66,422,058 cases recorded worldwide (as of 08-Dec-20). At the end of January 2020, the first cases were confirmed in Germany as well. Germany has had 1,197,709 cases so far (as of 08-Dec-20). The WHO declared a Public Health Emergency of International Concern on 2020-01-31.
SARS-CoV-2 and influenza viruses share some similarities. For instance, the primary route of transmission for both of these pathogens is the airborne route through
virus-laden respiratory droplets generated by breathing, coughing, talking, and sneezing. The symptoms in the early stage are typical for respiratory viral pathogens. The most commonly reported symptoms for both pathogens are fever, cough, and nasal congestion. Since SARS-CoV-2 disease courses can vary greatly in symptoms and severity (from asymptomatic to severe pneumonia with lung failure and death), the differentiation of SARS-CoV-2 and influenza viruses is important for further therapy.
Image Gallery
Accessories:
| Art. No. | PG6825 |
|---|---|
| Test format | real-time PCR with 200 reactions |
| Shelf life | 12 months from production |
| Sensitivity | ≥ 50 RNA copies per reaction |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.