Intended use:
For in vitro diagnostic use. RIDA®GENE Norovirus I & II is a real-time multiplex RT-PCR for the direct, qualitative detection and differentiation of norovirus (genogroup I and II) in human stool samples. RIDA®GENE Norovirus I & II real-time multiplex RT-PCR is intended for use as an aid in diagnosis of gastroenteritis caused by noroviruses.
General information:
Noroviruses cause by far the most cases of non-bacterial gastroenteritis outbreaks. A gastroenteritis caused by norovirus is manifested by severe nausea, vomiting and diarrhea. Noroviruses are egested by stool and with the vomit. An airborne transmission through aerosols containing the virus is often the cause of a very rapid spreading in shared facilities. The CDC estimates that more than 21 million cases of acute gastroenteritis, 70.000 hospitalisations and 800 deaths are caused by norovirus infections each year in the United States.
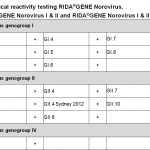
Noroviruses belong to the family of Caliciviridae. Because of their morphology (small round structured virus (SRSV)), they can be distinguished from the traditional calicivirus. The SRSVs were named after the place of their isolation. Thus, the name Norwalk-like stood for all viruses which have been isolated during outbreaks of gastroenteritis. The name originated from the first SRSV isolation in the city of Norwalk, Ohio, in the US in 1972. Later, other isolates like Snow Mountain agent, Hawaii agent and Montgomery County agent were named in a similar way. Noroviruses are small, non-enveloped viruses with a single-stranded RNA (ssRNA). They can be grouped in 7 genogroups with currently over 30 genotypes and a multiplicity of clades. So far, human pathogens have only been described from genogroup I (GI) with 9 genotypes, from genogroup II (GII) with 22 genotypes and from genogroup IV (GIV) with two genotypes.
Image Gallery
| Art. No. | PG1415 |
|---|---|
| Test format | real-time RT-PCR with 100 reactions |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.