Molecular detection methods have revolutionized medical diagnostics and are an essential part of today’s clinical laboratory routine.
In many areas of infectious diseases, the classical detection methods such as culture and immunoassays have been replaced or supplemented by real-time polymerase chain reaction (PCR).
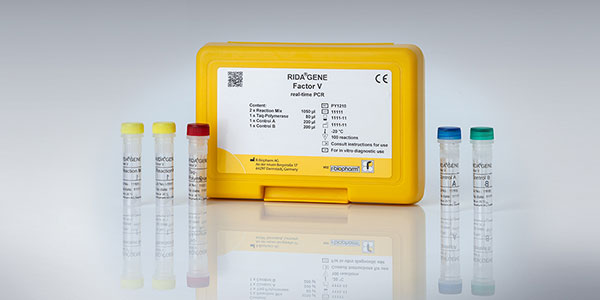
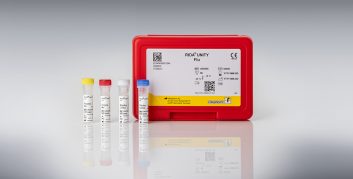
Real-time PCR – RIDA®GENE & RIDA®UNITY
By direct detection of DNA or RNA of the pathogen, real-time PCR allows for an early and highly specific diagnosis, thus leading to a fast and selective treatment regime of the individual patient; after DNA isolation, target gene fragments are amplified (amplicons) and, if present, detected with fluorescent hydrolysis probes. The fluorescence signal increases with the amount of formed amplicons which is detected by the optical unit of a real-time PCR instrument.
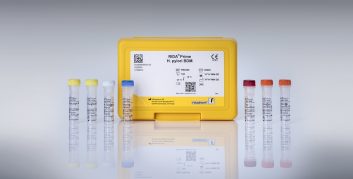
Multiplex Tandem PCR – TandemPlex®
MT-PCR offers unrivalled multiplexing capabilities and enables comprehensive and efficient diagnostics in the field of syndromic testing, among others The unique patented technology allows the detection of multiple targets in one sample without compromising analytical sensitivity and specificity. Method based on nested primer pairs ensure that only the relevant nucleic acids are amplified.
Choose an indication
Choose a diagnostic field
You might also be interested in
Real-time PCR support
Questions? Tap into our team’s expertise. We’re here to support you and your business throughout the testing process to ensure your success