Intended use:
For in vitro diagnostic use. RIDA®GENE EAEC is a multiplex real-time PCR for the direct, qualitative detection of enteroaggregative E. coli (EAEC) in human stool samples. RIDA®GENE EAEC multiplex real-time PCR is intended for use as an aid in diagnosis of gastroenteritis caused by enteroaggregative E. coli.
General information:
Escherichia coli (E. coli) are gram negative, facultatively anaerobic rod bacteria which move by peritrichal flagellation and belong to the Enterobacteriaceae family. E. coli are part of the normal intestinal flora of humans and many farm animals and are generally nonpathogenic. Some E. coli strains are pathogenic to humans through the acquisition of certain virulence factors (e.g. genes for toxins).
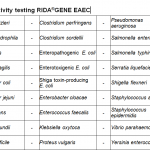
The six known intestinal pathogenic E. coli: enterohämorrhagic E. coli (EHEC), enteropathogenic E. coli (EPEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC) und diffusely adherent E. coli (DAEC) can be differentiated by the virulence factors.
Enteroaggregative E. coli (EAEC) were first identified and described in the stool of a child from Chile in 1987. The defining feature of EAEC is its characteristic aggregative adherence (AA) phenotype. In the gold standard HEp-2 cell adherence assay EAEC adhere to the epithelial cell surface in a “stacked-brick” formation. EAEC are defined as E. coli that do not secrete heat-labile or heat-stable enterotoxins and adhere to HEp-2 cells in an AA pattern. Certain EAEC strains carry a high molecular weight plasmid (pAA) associated with AA, on which a number of virulence genes (e.g. aggR, aggA, aafA, agg3 and aatA) are located. Important virulence genes for EAEC detection by PCR are the aatA gene (anti-aggregation protein transporter gene, referred to as CVD432 or EAEC probe) and the aggR gene (master regulator of the EAEC plasmid virulence genes). EAEC strains that carry pAA are regarded as typical EAEC while strains that lack pAA are regarded as atypical EAEC. The most common clinical manifestation of EAEC Infection is watery diarrhea. Less common associated clinical symptoms are low-grade fever, nausea, vomiting, abdominal pain and the presence of fecal blood, mucus or leukocytes. The incubation ranges from 8 – 18 h. A voluntary study with an inoculum of 1010 c.f.u. of EAEC caused diarrheal illness. Due to the high required infective dose suggests a fecal-oral transmission of EAEC by food or water.
EAEC is the cause of acute and chronic (> 14 days) diarrhea among children, adults and HIV-infected persons, in both developing and industrialized countries.
It is the second most common cause of travelers’ diarrhea after ETEC among travelers to developing countries, such as Mexico, India and Jamaica. Outbreaks of EHEC diarrhea have been reported and linked to the consumption of contaminated food. EAEC has been isolated from 2 % – 68 % of patients with diarrhea and from 0 % – 15 % of controls from India, South America, Europe and the Middle East. In a meta-analysis of published studies, EAEC was a cause of acute diarrhea in a median of 15 % of children living in developing countries and 4 % of children living in industrialized countries. EAEC were isolated from 2 % of pediatric patients with diarrhea in Germany compared with none of healthy controls. In Swiss children EAEC was isolated in 10.2 % of children with diarrhea compared with 2.2 % of children without diarrhea. In the US EAEC was isolated 4.5 % of case patients versus 1.7 % of controls.
Image Gallery
| Art. No. | PG2215 |
|---|---|
| Test format | real-time PCR with 100 reactions |
| Shelf life | 24 months after production |
| Sensitivity | Analytical Sensitivity EAEC: ≥ 10 DNA copies per reaction |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.