Intended use:
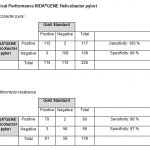
For in vitro diagnostic use. RIDA®GENE Helicobacter pylori is a multiplex real-time PCR for the direct, qualitative detection of Helicobacter pylori and its resistance to clarithromycin from human native tissue biopsy material.
The RIDA®GENE Helicobacter pylori multiplex real-time PCR is intended for use as an aid in diagnosis of gastric infections caused by Helicobacter pylori.
General information:
Helicobacter pylori (H. pylori) is a gram-negative rod-shaped bacterium which colonises the human gut. H. pylori increases the secretion of stomach acid and hence leads to different gastric infections such as Type B Gastritis, gastric ulcers or duodenal ulcers. Worldwide, H. pylori has a prevalence rate of 50 %, whereas the infection rate is higher in developing countries compared to developed countries. In Germany, about 33 million people are infected with H. pylori of which 10 – 20 % develop ulcers. While the H. pylori strain type 2 lacks the pathogenicity factors cag and VacA, an infection with H. pylori strain type 1 leads to gastroduodenal ulcers and in case of a chronic infection, significantly increases the risk of gastric cancer. To protect itself from gastric acid, H. pylori settles inside the gastric mucosa. Here, H. pylori splits urea by the enzyme urease to increase the pH value in its close surroundings.
Today, H. pylori is detected by microscopy or by using the helicobacter-urease assay from gastric biopsies. Other detection methods are antigen testing or breath tests.
After diagnosis of H. pylori, different treatment measures are possible. Often, the “Triple Therapy” is used which consists of a combination of Amoxicillin, Clarythromycin and a proton pump inhibitor or Metronidazol, Clarythromycin and a proton pump inhibitor. However, increasing clarithromycin resistance lowers the success rate of such a treatment by 30 %. Also, other more and more often occurring resistances against antibiotics such as Metronidazol or Levofloxacin (Fluoroquinolon) lead to higher failure in H. pylori eradication therapies.
Image Gallery
| Art. No. | PG2305 |
|---|---|
| Test format | real-time PCR with 100 reactions |
| Shelf life | 24 month after production |
| Sensitivity | ≥ 10 DNA copies per reaction |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.