Intended use:
For in vitro diagnostic use. RIDA®GENE Adenovirus is a multiplex real-time PCR for the direct, qualitative detection of Adenovirus in human stool samples, throat rinsing fluid, sputum and bronchoalveolar lavage (BAL).
The RIDA®GENE Adenovirus real-time PCR is intended for use as an aid in diagnosis of respiratory infections caused by adenoviruses.
General information:
Adenoviruses are non-enveloped ikosaedric double-stranded DNA (dsDNA) viruses and belong to the family of Adenoviridae. They were isolated from human pharyngeal tonsils (Adenoides), where their name originated from. One differentiates 56 serotypes of human adenoviruses and they are classified into seven groups (A – G). Adenoviruses cause a variety of different clinical pictures. Besides ocular and gastrointestinal infections, adenoviruses mostly cause respiratory disease. The latter one is primarily observed in small children under the age of four since they lack humoral immunity. However, 1 – 7 % of adult respiratory infections are caused by adenoviruses. The symptoms of an adenovirus infection reach from cold, acute bronchitis to pneumonia and in immunocompromised patients, also acute respiratory distress syndrome (ARDS) is observed. Acute respiratory infections are mainly caused by serotypes 1, 2, 3, 4, 6, 7, 14 and 21, whereas serotypes 1, 2, 3, 4 and 7 are the major causes of pneumonia. Many adenoviruses are endemic with adenovirus outbreaks being often described on military bases. In 2006/2007, a new adenovirus variant of serotype 14 lead to a major respiratory disease outbreak with a mortality rate of 5%. The clinical picture of an adenovirus infection is also dependent on its viral entry to the host. Inhalation with adenovirus 7 leads to major infection of the lower respiratory tract but oral uptake of adenovirus 7, if at all, may only lead to mild infection. The RIDA®GENE Adenovirus assay was developed as an aid in diagnosis of respiratory infections, although Adenovirus serotypes, which cause primarily gastrointestinal infections (serotype 40 and 41), can be detected from stool samples.
Image Gallery
Accessories:
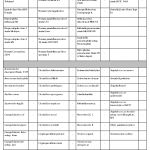
| Art. No. | PG1005 |
|---|---|
| Test format | real-time PCR with 100 reactions |
| Shelf life | 24 months after production |
| Sensitivity | ≥ 10 DNA copies per reaction |
Dear customers,
we have started to provide the documents for our products in an electronic format. These are the Instructions for Use (IFU), the Safety Data Sheets (SDS) and the Certificate of Analysis (CoA). For batches placed on the market after 01 January 2023, you can find our documents on the eIFU portal eifu.r-biopharm.com/clinic.